
|
D113 /
Safety Related Properties Arising From Hydrogens Atomic StructureSafety related properties arising from hydrogens atomic structureThe hydrogen molecule exists in two forms, distinguished by the relative rotation of the nuclear spin of the individual atoms. These distinct forms are known as ortho-hydrogen and para-hydrogen and the following should be noted when dealing with the safety of hydrogen:
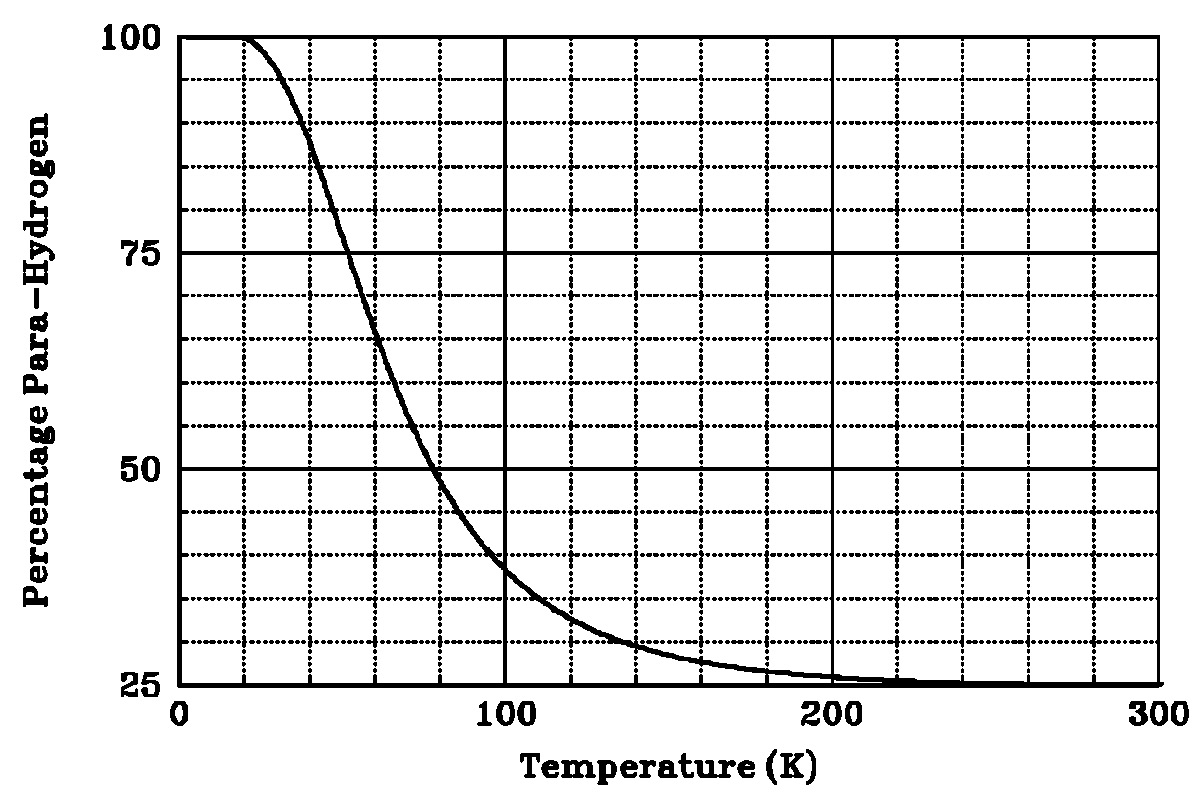 Figure 1: Equilibrium percentage of para-hydrogen versus temperature [21]. The ortho- to para-conversion is accompanied by the release of heat. It is known to be a slow process (taking several days) and the value of the heat effect depends on the temperature at which it occurs (see Figure 2). The heat release may complicate low temperature hydrogen processes, particularly liquefaction. Catalysts are used to accelerate this conversion in liquid hydrogen production facilities, which produce para-hydrogen liquid with a purity of more than 95 percent. If unconverted liquid hydrogen is stored at its boiling point 20 K (minus -253 şC) the ortho-hydrogen is slowly converted to para-hydrogen. The rate of the non-catalytic conversion is given by [25] 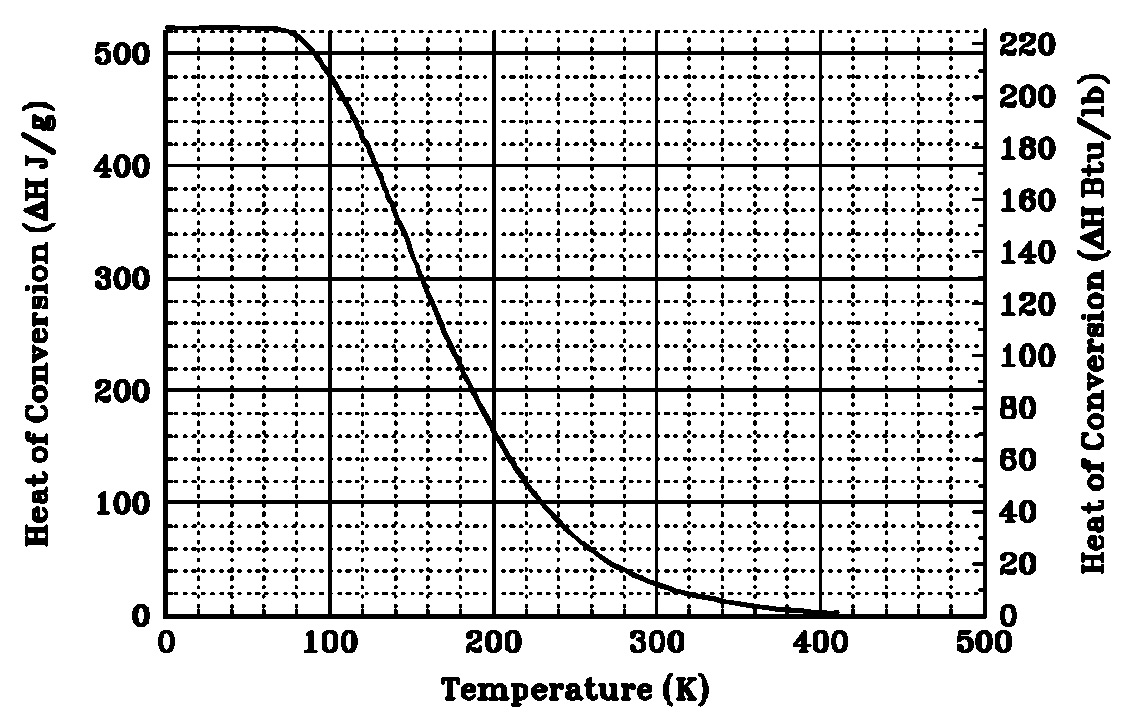 Figure 2: Heat release during the conversion of normal hydrogen [21]. [21] NASA. Safety standard for hydrogen and hydrogen systems. Guidelines for hydrogen system design, materials selection, operations, storage, and transportation. Technical Report NSS 1740.16, Office of Safety and Mission Assurance, Washington, 1997. [25] Zabetakis M.G. and Burgess D.S. Research on the hazards associated with the production and handling of liquid hydrogen. Bureau of Mines Report of Investigation RI 5707, US Department of Interior, 1961. [26] Fay J.A. Model of spills and fires from LNG and oil tankers. Journal of Hazardous Materials, B96:171-188, 2003. [27] Verfondern K. and Dienhart B. Experimental and theoretical investigation of hydrogen pool spreading and vaporization. International Journal of Hydrogen Energy, 22:649-660, 1997. Density of hydrogen?Buoyancy of gaseous hydrogen?Buoyancy of liquid hydrogen?Correlations for the density of hydrogen? |