
|
BRHS /
Transport Of Liquid HydrogenIn order to reduce the volume required to store a useful amount of hydrogen - particularly for vehicles - liquefaction may be employed. Since hydrogen does not liquefy until it reaches ÷253°C (20 degrees above absolute zero), the process is both time consuming and energy demanding. Critical temperature for hydrogen is T=-240°C. This is the upper limit for liquefaction, at higher temperatures it is indeed impossible. T=-253°C is the equilibrium temperature at p=1bara. The advantage of liquid hydrogen is its high energy/mass ratio, three times that of gasoline. It is the most energy dense fuel in use (excluding nuclear fuels), which is why it is employed in all space programmes. However, energy/volume ratio remains a challenge. Liquid hydrogen is difficult to store over a long period (product loss by vaporisation), and the insulated tank required may be large and bulky. An illustration comparing energy densities between gasoline, diesel and hydrogen is shown in figure 1 below. 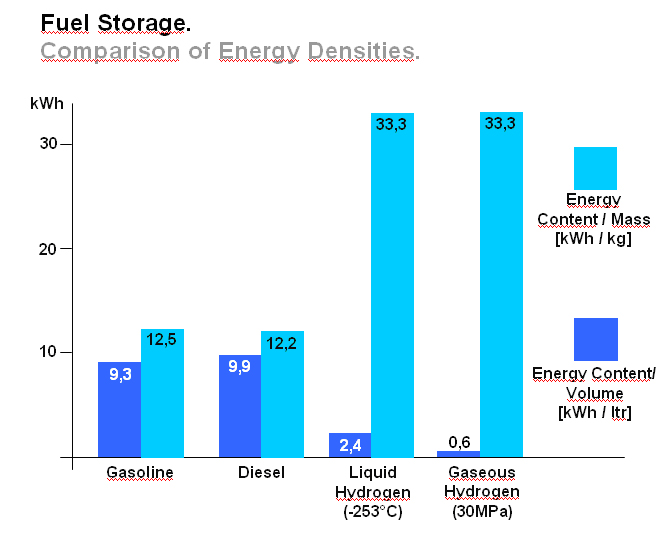 Figure 1: Fuel Storage. Comparison of energy densities between gasoline, diesel and hydrogen, (figure from [BMW]) Liquid hydrogen road transport is carried out using trucks which can exceed a capacity of 60000 litres. Delivery is achieved either in vacuum insulated containers or by transferring the product to stationary vessels depending on the required quantities. In the USA there are several pipelines for liquid hydrogen with lengths of up to 40 km iii. The intercontinental transport of hydrogen will probably be carried out in liquid form using ships. For this purpose, specialized ships with appropriate tanks and port facilities are being designed. A realization of these ideas will however not take place until the trade in hydrogen reaches an appropriately large scale. << Transport of gaseous hydrogen | Content | Transport in compound materials >> |