
|
BRHS /
Experimental Work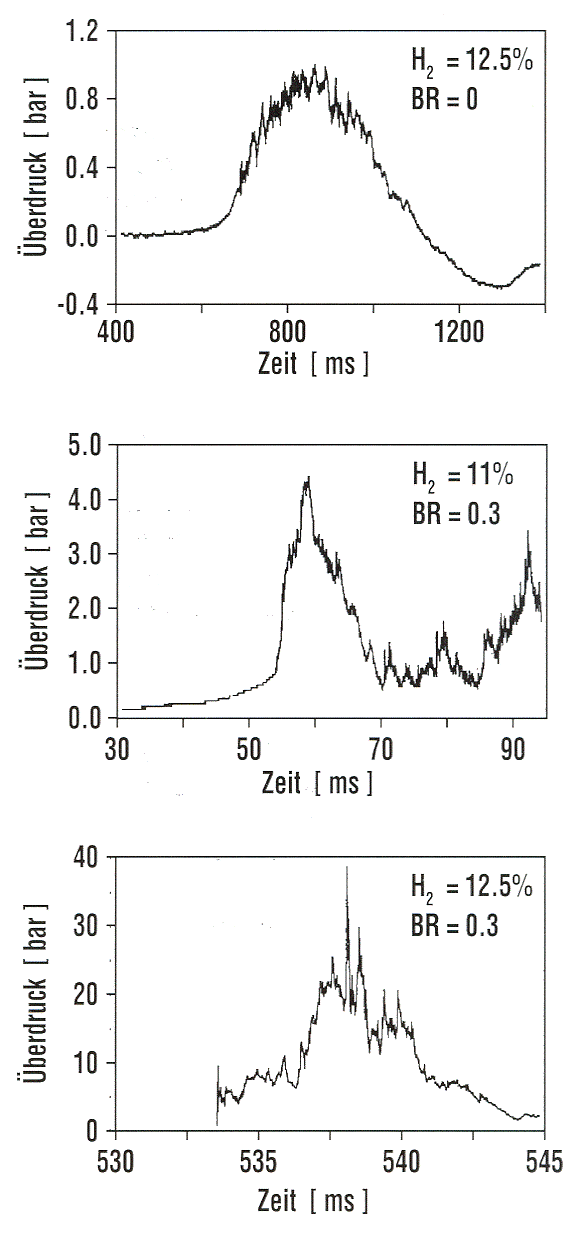 Fig. 1: Measured overpressure transients in RUT tests with different blockage ratios (BR) showing slow deflagration (top), fast deflagration (middle), detonation (bottom) ([[http://hysafe.org/wiki/BRHS/ExperimentalWork?action=bibentry&bibfile=DB&bibref=BreitungW:1996 | BreitungW:1996)]] Apart from the experience obtained by observations and lessons learned from explosion accidents, numerous experiments have been performed worldwide to investigate the transient behavior of overpressures following the explosive combustion of fuel-air mixtures. Tests were conducted under various conditions such as confined, partially confined, or unconfined, larger-scale or smaller-scale geometry, fuel type and constitution with the main goal of development of or comparison with simulation approaches. The most dangerous configurations were found to be, as expected, those with a major obstruction, even for less sensitive fuel gases such as methane. For DDT cases, travelling distance for the flame must be sufficiently long, which would be around 3 m for a stoichiometric hydrogen-air mixture. Only a few of those test series are mentioned in the following showing the broad range of activities. Large-scale experiments were conducted by the Russian Kurchatov Institute using premixed hydrogen-air mixtures. The RUT facility with a confined volume of 480 m3 was employed for a series of tests ranging from slow deflagration to detonation. Hydrogen concentrations varied between 10 and 14 %. During slow deflagration (no obstacles present), the overpressures measured increased with H2 concentration, from around 0.1 MPa to 0.17-0.23 MPa. Insertion of obstacles (blockage ratio of 30 and 60 %) resulted in accelerated flames creating overpressures of 1.1-1.6 MPa for gas mixtures with 14 % H2 concentration. There was even the observation of a detonation at a H2 concentration as low as 12.5 % (Fig. 1). The Russian UTR facility, a tube with 66 mm diameter and a maximum length of 3 m, was used for systematic studies with gas mixtures of hydrogen, oxygen, and nitrogen. Peak overpressures and impulses were investigated as a function of the location of DDT which influences the degree of precombustion. Peak pressures observed were well above the Chapman-Jouguet pressures for detonation of the undisturbed mixture (BreitungW:1996). Large-scale testing on DDT in hydrogen-air mixtures was conducted in the FLAME facility ("Flame Acceleration Measurements and Experiments"), a 30.5 m long, 2.44 m high, and 1.83 m wide rectangular channel with a closed ignition end and an open far end and with venting/obstruction possibilities between fully closed and 50 % open. Tests were carried out with variation of H2 concentrations (12-30 vol%), obstacle blockage ratio (0-33 %), and top venting. The observed lower detonability limits were at 15 vol% of H2 with obstacles and 25 vol% of H2 without obstacles. DDT also occurred at a 24.8 vol% H2 concentration with a 13 % open top. Top venting was found to have two counteracting effects: increase of burning rate due to turbulence dominant at low venting rates, and flame speed reduction due to loss of gas for large venting rates (YangJW:1991). An explosion tube of 2.5 m diameter and 10 m length with one open end was used in Norway to study peak overpressures of ignited stoichiometric propane-air mixtures. The tests have shown the significant influence of the blockage ratio inside the tube on the flame speed and pressure increase, respectively, which can come close to the detonation range (GexCon:2006). Smaller-scale detonation test tubes have been conducted at the Research Center Karlsruhe, the Technical University of Munich, the DLR in Stuttgart or the High-Temperature Combustion Facility, HTCF, at BNL employing different types of obstruction and differently diluted hydrogen-air mixtures to study flame acceleration and various DDT mechanisms. Within the nuclear power plant safety program and the PNP gas cloud program, the German Fraunhofer Institute for Chemical Technology (FH-ICT) conducted various series of tests using mixtures of propane, ethylene, methane, and hydrogen with air to investigate detonation and DDT in spherical, hemispherical, and tube geometries. Unconfined hemisperically shaped H2-air mixtures at volumes between 7.5 and 2100 m3 were ignited measuring a maximum overpressure of 6.3 kPa which corresponds to a flame velocity of 84 m/s (SchneiderH:1978); (PfortnerH:1983); (PfortnerH:1983a); (PfortnerH:1985). Balloon tests were conducted with hemisperically shaped H2-air mixtures with a volume of 50 m3 and concentrations of 20 and 29.6 vol%, respectively. Ignition occurred at the center on the ground by means of an explosive to trigger detonation. Pressures were measured at various positions inside and outside the balloon (Figs. 2 and 3). Visually measured flame speeds agreed well the theoretical values (BreitungW:1995).  Fig. 2: Measured and calculated pressure transient inside the balloon in an FH-ICT hemispherical balloon test with H2-air detonation ([[http://hysafe.org/wiki/BRHS/ExperimentalWork?action=bibentry&bibfile=DB&bibref=BreitungW:1995 | BreitungW:1995)]] 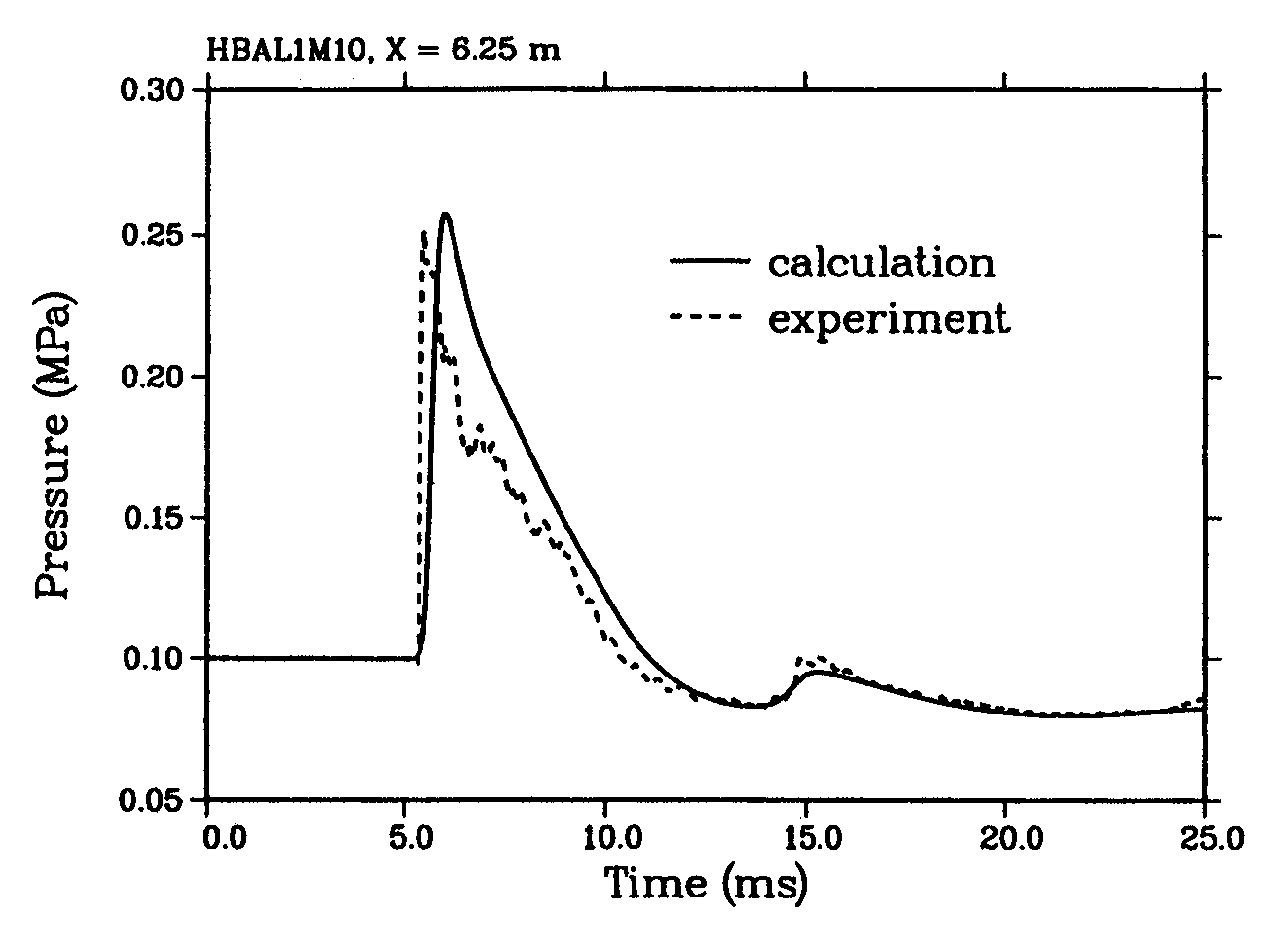 Fig. 3: Measured and calculated pressure transient outside the balloon in an FH-ICT hemispherical balloon test with H2-air detonation ([[http://hysafe.org/wiki/BRHS/ExperimentalWork?action=bibentry&bibfile=DB&bibref=BreitungW:1995 | BreitungW:1995)]] The influence of partial confinement on the combustion behavior of H2-air mixtures was examined in further ICT tests employing a 10 x 3 x 3 m3 lane with parallel walls (PfortnerH:1983); (PfortnerH:1983a); (SchneiderH:2005), which also have resulted in one case in a transition from deflagration to detonation (Fig. 4). The extensive experimental research programs on gas explosions within the EU projects MERGE (MercxWPM:1994) and EMERGE (MercxWPM:1997) have shown that overpressures are mainly determined by fuel type, geometric scale as well as the arrangement and number of obstacles which are passed by the propagating flame. Other unconfined explosion tests are known of the BASF company in Germany. In 1943 and 1948, explosion accidents occurred at BASF resulting from the bursting of liquid gas vessels, subsequent flash evaporation, and mixing of the fuel with ambient air and eventually ignition of the cloud. The cause of the bursting was a heating of the overfilled vessels by the radiation of the sun, i.e., there was not enough vapor buffering inside of the tanks. The experimental simulation and modeling of these events has been performed in the 1970s by BASF and Fraunhofer ICT by use of differently sized vessels with volumes between 0.2 and 1.2 m3 corresponding to a mass of up to 452 kg of liquid propylene. Pressures observed were in the range of 0.5-1.5 kPa for the smaller and 4-7 kPa for the larger vessels (MaurerB:1975); (MaurerB:1977); (GiesbrechtH:1980); (GiesbrechtH:1981). With respect to other physical explosions, tests were conducted in the 1970s and 1980s with the spillage of LNG into a pond of water (e.g., Coyote series, Burro series, Maplin Sands series) to measure among other parameters the strength of RPT pressure waves. After releasing LNG amounts of 40 m3 onto water, observed RPT overpressures were as high as 5 kPa (KoopmanRP:1982). 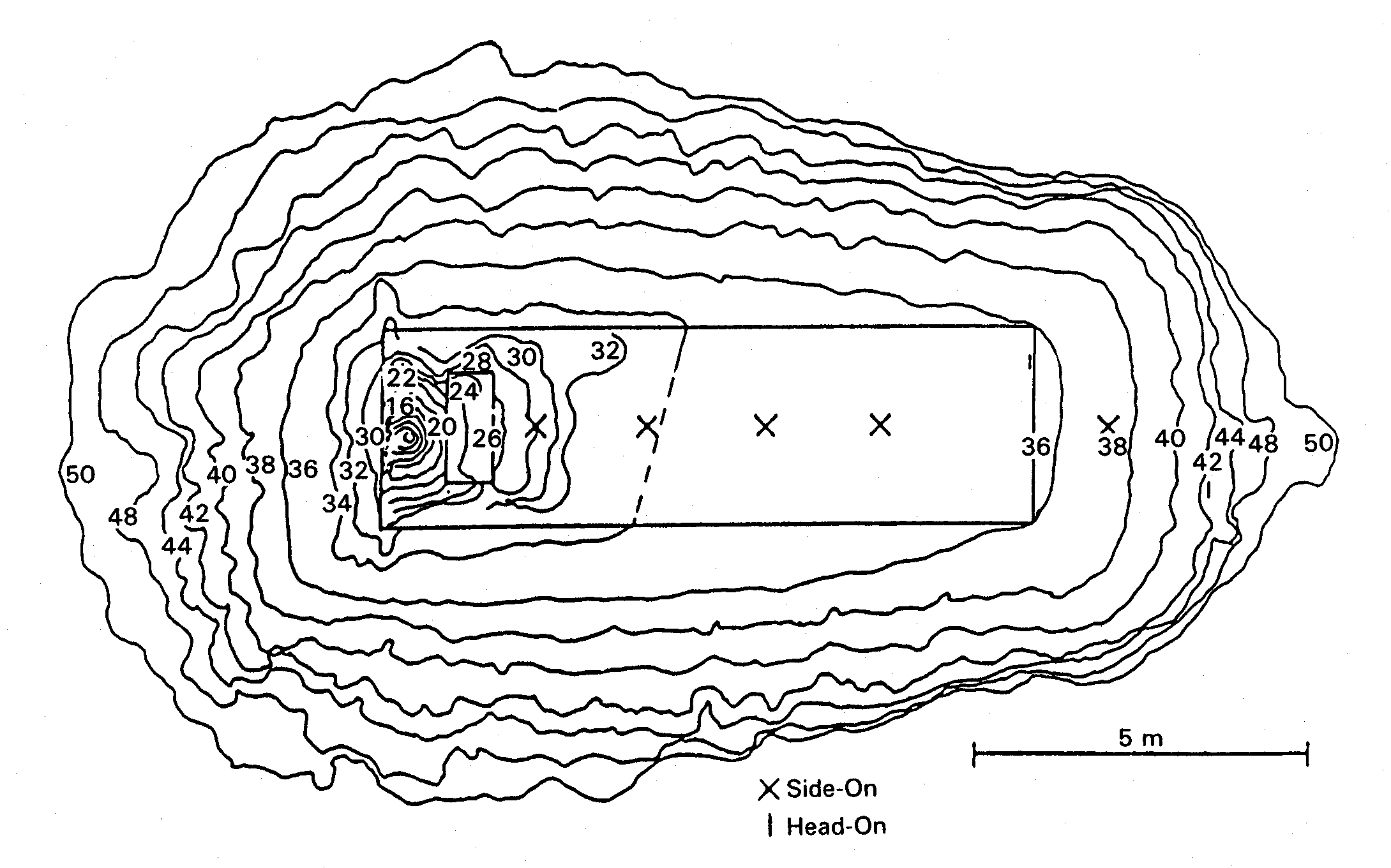 Fig. 4: Measured flame front profiles in an FH-ICT lane test with parallel walls (37 % H2 air mixture), fan generated turbulence, and DDT near the wall (contour 34) ([[http://hysafe.org/wiki/BRHS/ExperimentalWork?action=bibentry&bibfile=DB&bibref=BermanM:1986 | BermanM:1986)]] Gaz de France initiated an RPT research program in 1981 in Lorient with large-scale tests using LNG. The spillage of amounts between 1 and 9 m3 onto water has shown that the occurrence and strength of RPT were strongly related to the volume of the mixing zone. Maximum explosion pressure recorded was equivalent to 4.15 kg of TNT. Research activities also included fundamental studies of the phenomena and computer code development. Due to the larger temperature difference, consequences of LH2 spills onto water may be more severe. Invalid BibTex Entry! << Physical Explosions | Content | Modeling of Pressure Waves >> |